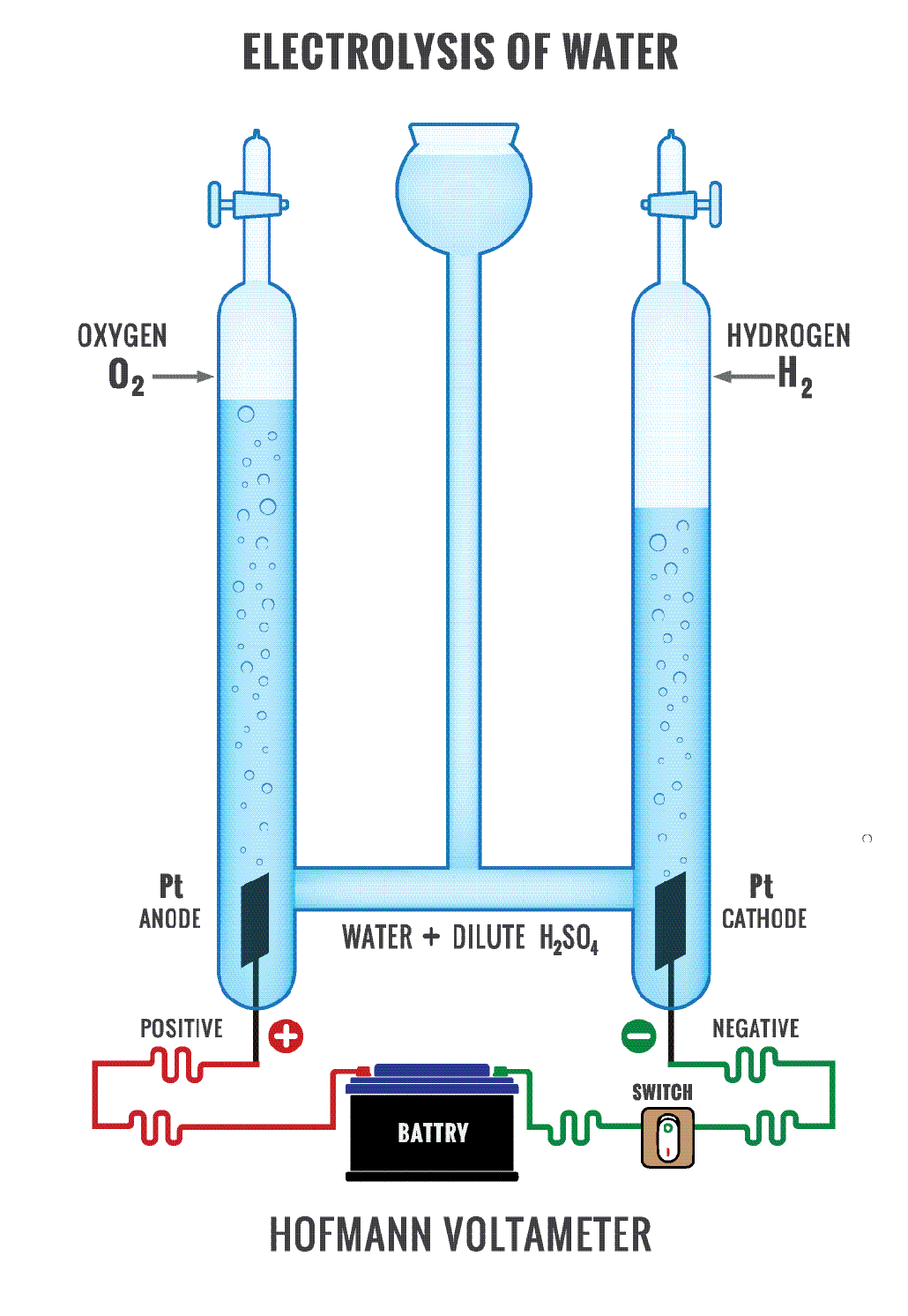
What Is Difference Between Electrochemical Cell And Electrolytic Cell . In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. The main differences are outlined below: An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In an electrochemical cell, half cells are placed in different containers and connected. However, there are also striking differences between the two cells. Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. The chemical reaction that occurs inside such cells is commonly referred to. This is the only fundamental difference between an electrolytic cell and the galvanic cell in which the free energy supplied by the cell reaction is. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell.
from studylibuncoping.z4..core.windows.net
Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. In an electrochemical cell, half cells are placed in different containers and connected. The chemical reaction that occurs inside such cells is commonly referred to. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. However, there are also striking differences between the two cells. The main differences are outlined below: This is the only fundamental difference between an electrolytic cell and the galvanic cell in which the free energy supplied by the cell reaction is.
Why Are Inert Electrodes Used In Electrolysis
What Is Difference Between Electrochemical Cell And Electrolytic Cell The chemical reaction that occurs inside such cells is commonly referred to. The chemical reaction that occurs inside such cells is commonly referred to. However, there are also striking differences between the two cells. Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In an electrochemical cell, half cells are placed in different containers and connected. This is the only fundamental difference between an electrolytic cell and the galvanic cell in which the free energy supplied by the cell reaction is. The main differences are outlined below: In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content What Is Difference Between Electrochemical Cell And Electrolytic Cell In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. The main differences are outlined below: However, there are also striking differences between the two cells. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In an electrochemical. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From circuitdelgerf1.z21..core.windows.net
What Happens At The Cathode In Electrolysis What Is Difference Between Electrochemical Cell And Electrolytic Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. This is the only fundamental difference between an electrolytic cell and the galvanic cell in which the free energy supplied by the cell reaction is. However, there are also striking differences between the two cells. The most significant. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell What Is Difference Between Electrochemical Cell And Electrolytic Cell Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. However, there are also striking differences between the two cells. The main differences are outlined below: This is the. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From www.pinterest.com
Difference Between Electrochemical Cell and Electrolytic Cell What Is Difference Between Electrochemical Cell And Electrolytic Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In an electrochemical cell, half cells are placed in different containers and connected. Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. However, there are also striking differences between the. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell What Is Difference Between Electrochemical Cell And Electrolytic Cell This is the only fundamental difference between an electrolytic cell and the galvanic cell in which the free energy supplied by the cell reaction is. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In this tutorial on galvanic cells, aka voltaic cells, you will learn the. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From www.vrogue.co
Electrochemical Cells Vrogue What Is Difference Between Electrochemical Cell And Electrolytic Cell In an electrochemical cell, half cells are placed in different containers and connected. Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In this tutorial on galvanic cells, aka voltaic cells, you will learn the. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From www.difference.wiki
Daniel Cell vs. Leclanche Cell What’s the Difference? What Is Difference Between Electrochemical Cell And Electrolytic Cell The chemical reaction that occurs inside such cells is commonly referred to. Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In an electrochemical cell, half cells are placed in different containers and connected. The. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From www.differencebetween.com
Difference Between Electrochemical Cell and Galvanic Cell Compare the What Is Difference Between Electrochemical Cell And Electrolytic Cell However, there are also striking differences between the two cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. The chemical reaction that. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From circuitwiringsimis101.z19..core.windows.net
What Happens At The Cathode In Electrolysis What Is Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. However, there are also striking differences between the two cells. The most significant difference between. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
The differences between the voltaic cell and the electrolytic cell What Is Difference Between Electrochemical Cell And Electrolytic Cell Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. This is the only fundamental difference between an electrolytic cell and the galvanic cell in which the free energy supplied by the cell reaction is. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From mavink.com
Diagram Of Electrochemical Cell What Is Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. In an electrochemical cell, half cells are placed in different containers and connected. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. An apparatus that is used to generate. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From www.youtube.com
Comparison between the electrolytic cell and chemical cell YouTube What Is Difference Between Electrochemical Cell And Electrolytic Cell The chemical reaction that occurs inside such cells is commonly referred to. Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. However, there are also striking differences between the two cells. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In this tutorial on. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From www.pinterest.pt
Galvanic Cell and Electrolytic Cell Electrochemical Cells Teaching What Is Difference Between Electrochemical Cell And Electrolytic Cell However, there are also striking differences between the two cells. Electrolytic cells are a class of electrochemical cells that use electric currents to facilitate the cell reaction. The chemical reaction that occurs inside such cells is commonly referred to. In an electrochemical cell, half cells are placed in different containers and connected. An apparatus that is used to generate electricity. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From circuitwiringsimis101.z19..core.windows.net
What Happens At The Cathode In Electrolysis What Is Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: The chemical reaction that occurs inside such cells is commonly referred to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In an electrochemical cell, half cells are placed in different containers and connected. This is the only fundamental difference between. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell What Is Difference Between Electrochemical Cell And Electrolytic Cell In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. However, there are also striking differences between the two cells. This is the only fundamental difference between an electrolytic cell and the galvanic cell in which the free energy supplied by the cell reaction is. In. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science What Is Difference Between Electrochemical Cell And Electrolytic Cell This is the only fundamental difference between an electrolytic cell and the galvanic cell in which the free energy supplied by the cell reaction is. In an electrochemical cell, half cells are placed in different containers and connected. In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From wirelistsolfatara.z21..core.windows.net
Dry Cell Car Battery Vs Wet Cell What Is Difference Between Electrochemical Cell And Electrolytic Cell The main differences are outlined below: The chemical reaction that occurs inside such cells is commonly referred to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. However, there are also striking differences between the two cells. In this tutorial on galvanic cells, aka voltaic cells, you. What Is Difference Between Electrochemical Cell And Electrolytic Cell.
From pediaa.com
Difference Between Galvanic and Electrolytic Cell Definition, How What Is Difference Between Electrochemical Cell And Electrolytic Cell In this tutorial on galvanic cells, aka voltaic cells, you will learn the basics of redox reactions and how to apply this information to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. The most significant difference between an electrolytic cell and an electrochemical cell is that. What Is Difference Between Electrochemical Cell And Electrolytic Cell.